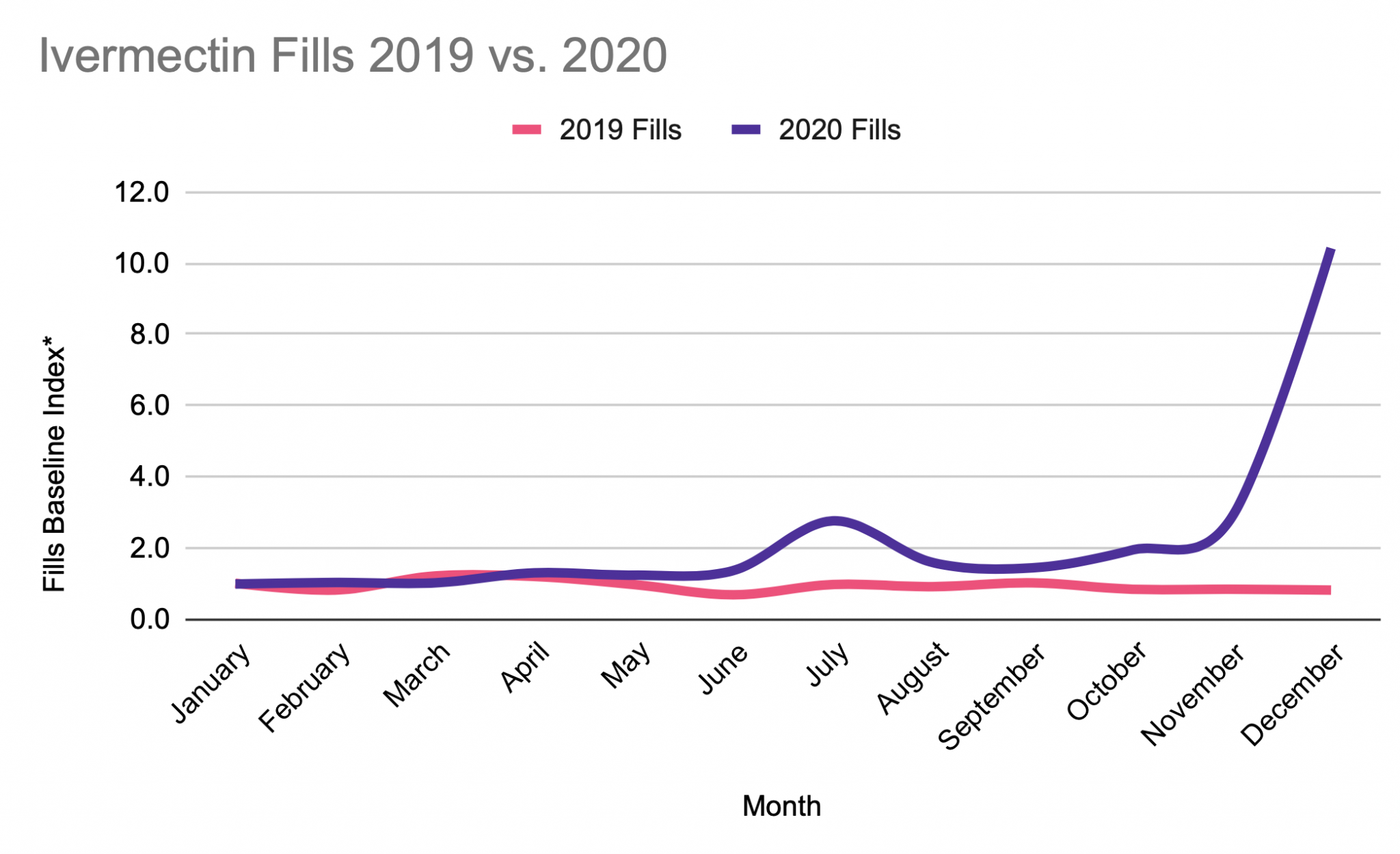
* Prescription savings vary by prescription and by pharmacy, and may reach up to 80% off cash price.
Pharmacy names, logos, brands, and other trademarks are the property of their respective owners.
This is a prescription discount plan. This is NOT insurance nor a Medicare prescription drug plan. The range of prescription discounts provided under this discount plan will vary depending on the prescription and pharmacy where the prescription is purchased and can be up to 80% off the cash price. You are fully responsible for paying your prescriptions at the pharmacy at the time of service, but you will be entitled to receive a discount from the pharmacy in accordance with the specific pre-negotiated discounted rate schedule. Pharmacy names, logos, brands, and other trademarks are the property of their respective owners.Towers Administrators LLC (operating as 'SingleCare Administrators') is the authorized prescription discount plan organization with its administrative office located at 4510 Cox Road, Suite 111, Glen Allen, VA 23060. SingleCare Services LLC ('SingleCare') is the vendor of the prescription discount plan, including their website.website at www.singlecare.com. For additional information, including an up-to-date list of pharmacies, or assistance with any problems related to this prescription drug discount plan, please contact customer service toll free at 844-234-3057, 24 hours a day, 7 days a week (except major holidays). By using the SingleCare prescription discount card or app, you agree to the SingleCare Terms and Conditions found at https://www.singlecare.com/terms-and-conditions