More than 200 million COVID-19 vaccines have been administered in the United States and as of Apr. 19th, the U.S. government opened vaccine eligibility to everyone over the age of 16 in all 50 states. With three vaccines currently approved by the Food and Drug Administration under the Emergency Use Authorization, those newly eligible to access vaccines may wonder which are the most effective. Understanding each aspect of each vaccine can be confusing—especially when there is misinformation circulating such as the myths that the vaccine causes infertility or gives you COVID-19.
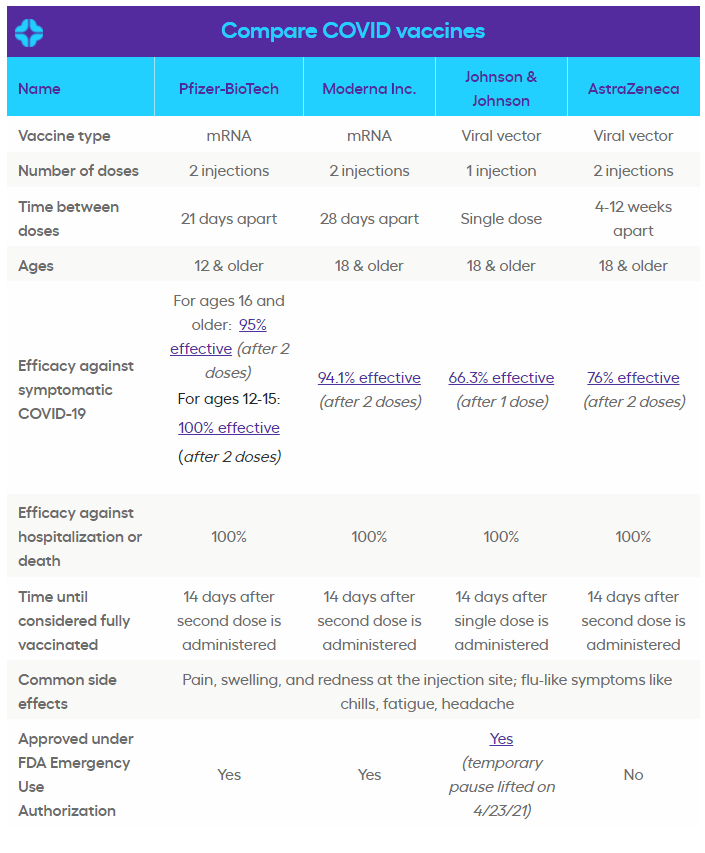
As Americans race to secure vaccine appointments, SingleCare put together a comprehensive chart comparing the vaccines to one another. In addition, Dr. Ramzi Yacoub, Pharm. D., and chief pharmacy officer at SingleCare, answers frequently asked questions about the COVID vaccines.
Coronavirus vaccine FAQs
What’s a viral vector vaccine vs. a messenger RNA (mRNA) vaccine?
Dr. Yacoub: “Vaccines have all different types of technologies that help trigger your body’s immune system to respond to the virus in order to build antibodies. The COVID-19 vaccines primarily use two technologies, messenger RNA (mRNA) and viral vectors. mRNA, which is a new vaccine type used by the Pfizer and Moderna COVID vaccines, teaches human cells how to make a spike protein which triggers the immune response to then create antibodies against the virus. Viral vectors, which is used by the Johnson & Johnson and AstraZeneca vaccines, is a modified version of a different virus used to deliver instructions to our cells in order to create antibodies against the virus. Both mRNA and viral vector vaccines cannot and will not give you COVID-19. They do not interact with or affect DNA.”
What does efficacy mean and what should I look for?
Dr. Yacoub: “Most consumers look at the efficacy against symptomatic COVID-19, which is beneficial, however, it is not the efficacy that consumers should be most concerned with as these tend to differ solely based on when clinical trials occur. Since Pfizer and Moderna ran their vaccine trials in the summer of 2020 and Johnson & Johnson in the fall of 2020, you can’t compare this efficacy rate to each other as their trials weren’t conducted head to head. The efficacy all consumers should look for when getting a COVID-19 is against severe illness, hospitalization and death—which all three vaccines approved for EUA have 100% efficacy for.”
Could I get COVID-19 even though I received the first dose?
Dr. Yacoub: “Even though you may have received the first dose of the COVID-19 vaccine, you are not considered to be fully vaccinated until 2 weeks after your last dose of the vaccine. During this time, your immune system is responding to the vaccine and is hard at work to develop antibodies. If you are exposed to the virus in between doses or before the end of two weeks after your last dose, there is a possibility that you could contract COVID-19 as you are not completely protected against the virus.”
What are common side effects for each vaccine?
Dr. Yacoub: “Common side effects for all the COVID-19 vaccines include pain, swelling, and redness at the injection site and flu-like symptoms like chills, fatigue, and headache, that typically last no longer than 24 hours. For the Johnson & Johnson vaccine, 6 women experienced blood clots, which is a very rare reaction that caused the FDA to pause distribution for a short period. This reaction hasn’t been seen in either the Moderna or Pfizer COVID vaccines. If you received the Johnson & Johnson vaccine and are experiencing severe headache, leg or abdominal pain, or shortness of breath, you should contact your healthcare provider immediately or seek emergency medical attention.”
Will my vaccine protect me against new variants?
Dr. Yacoub: “There currently is limited information available on the efficacy of each of the vaccines against the different variants circulating in the country. However, according to the World Health Organization (WHO), the approved vaccines in the United States are expected to provide at least some protection against the new variants of the virus. As variants circulate, while rare there is the possibility for them to “break through” fully vaccinated people, making it even more important for people to receive the COVID vaccine. As more people get the vaccine, there is less of an opportunity for the virus to mutate, create variants and prolong the pandemic.”
Will each vaccine require booster shots?
Dr. Yacoub: “Recently, both CEOs of Moderna and Pfizer vaccine manufacturers said it’s likely that consumers will need a third “booster” shot within the first year of receiving their COVID-19 vaccine. However, the best indicator whether this will be required is simply time. Once we’ve gathered more information and data on the vaccine efficacy itself in real-life situations, it will determine if and when a booster vaccine is required.”
What are the side effects kids and teens can expect with the Pfizer COVID-19 vaccine?
Dr. Yacoub: “According to the FDA, those in the Pfizer clinical trial of 12-15 year olds experienced side effects that included injection site pain, fever, chills, headache, muscle aches and fatigue, primarily after their second dose which lasted 1-3 days. Before receiving the vaccine, adolescents should stay hydrated and drink plenty of water before and after their vaccination. If they do experience any side effects, they can take over-the-counter pain medications, like Tylenol or acetaminophen, to help alleviate any symptoms.”
Is the Pfizer vaccine for adolescents the same as adults?
Dr. Yacoub: “Yes, the Pfizer vaccine is the same across all age groups as it requires 2 doses, 21 days apart, for vaccination. Just like in adults, adolescents will not be considered fully vaccinated until 14 days after their second dose.”
Can anyone get the Pfizer vaccine?
Dr. Yacoub: “Currently, the FDA has approved the Pfizer COVID-19 vaccine for Emergency Use Authorization for those over the age of 12. Pfizer is conducting pediatric testing for ages 2-11 years and plans to submit to the FDA for emergency use approval by September.”
